A comparative analysis titled “Drug Risk Profile Comparison: AE Reporting for Two Common NSAIDs Ibuprufen vs. Naproxen”
A comparative analysis titled was conducted to evaluate differences in adverse event (AE) patterns between two widely used analgesics NSAIDs Ibuprufen vs. Naproxen.
ALL BLOGSMY PV PROJECTS
11/2/20251 min read
A comparative analysis titled “Drug Risk Profile Comparison: AE Reporting for Two Common NSAIDs Ibuprufen vs. Naproxen” was conducted to evaluate differences in adverse event (AE) patterns between two widely used analgesics.
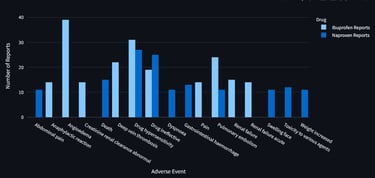
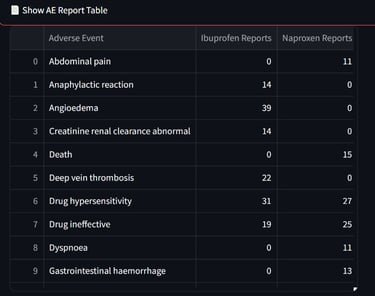
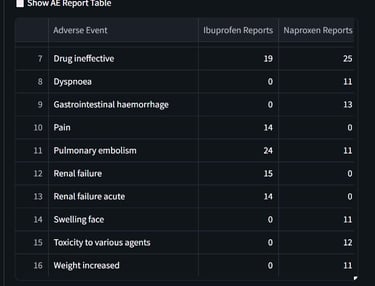
An interactive dashboard was developed to retrieve and analyze up to 1,000 post-marketing safety reports per drug from publicly available regulatory data. The tool enables real-time comparison of the top reported adverse events, highlighting both overlapping and drug-specific safety signals.
This resource supports data-informed pharmacovigilance by offering a streamlined method to explore AE profiles across similar therapeutic agents.