Analyzing Top adverse effects related to Salbutamol (openFDA resources)
Analyzing Top adverse effects related to Salbutamol (openFDA resources)- Extracted & Visualized into a bar plot the Top 20 reported AE by frequency .
MY PV PROJECTSALL BLOGS
11/2/20251 min read
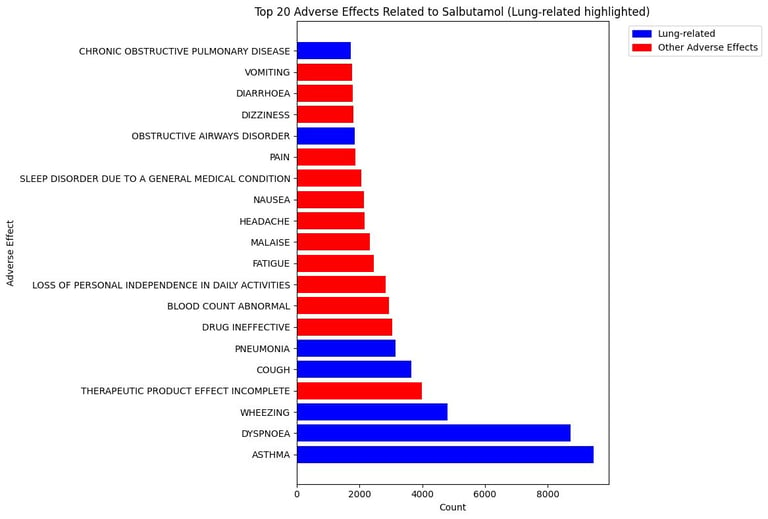
Interpretation of the Salbutamol Adverse Effects Analysis
1. High Frequency of Lung-Related Adverse Effects
The top 3 adverse events: asthma, dyspnoea, and wheezing are clearly lung-related
This makes sense because Salbutamol is a bronchodilator used to treat respiratory conditions like asthma and COPD.
- The high count of these terms may not always signal negative outcomes some might reflect underlying disease exacerbations or reporting bias in respiratory patients.
Interpretation:
- These reports may indicate treatment failure, disease progression, or improper use (e.g., overuse or incorrect inhalation technique), which require further clinical investigation.
2. Non-Respiratory Adverse Effects Appear Frequently Too
Effects like headache, nausea, fatigue, and dizziness are among the top reported (shown in red).
- These are known systemic side effects of β2-agonists like Salbutamol, especially when used in higher doses or absorbed systemically.
Interpretation:
- These may be true drug-related effects, consistent with the known pharmacological action of Salbutamol (e.g., stimulation of Beta 2-receptors in skeletal muscle and CNS).
3. Reports Like “Drug Ineffective” and “Therapeutic Product Effect Incomplete”
These terms reflect patient-reported treatment outcomes and lack of efficacy rather than side effects per se.
Interpretation:
They may indicate either improper drug use, resistance, disease worsening, or unmet treatment expectations and should be flagged for signal evaluation.
4. Clinical and Regulatory Relevance
This type of analysis supports signal detection, benefit-risk assessments, and pharmacovigilance planning.
By distinguishing between expected (on-label) and unexpected or serious reactions, teams can prioritize safety concerns or initiate risk mitigation.
--------------
How this analysis is beneficial:
- Regulatory & Drug Safety Teams: Helps monitor real-world evidence of drug safety and supports early signal detection.
- Medical Affairs & Clinical Teams: Enhances understanding of the drug's risk profile in diverse populations.
- Pharma Consultants & Data Analysts: Demonstrates practical applications of pharmacovigilance data science tools.
- Product Lifecycle Management: Informs benefit-risk evaluation and post-marketing surveillance strategy.
Key Insight: Lung-related effects such as asthma, dyspnea, and wheezing are the most frequently reported, aligning with the drug's primary site of action and known safety profile
.
This Analysis bridges pharmacological knowledge with real-world data insights